BeiGene Presents Results from SEQUOIA Trial of BRUKINSA (zanubrutinib) in First-Line Chronic Lymphocytic Leukemia at the 63rd ASH Annual Meeting
BRUKINSA demonstrated superiority in progression-free survival over chemoimmunotherapy as a first-line treatment for patients with chronic lymphocytic leukemia
Consistent efficacy was observed across high-risk patient subgroups
Safety results were generally consistent with its previously reported profile
Preliminary safety and efficacy data from Cohort 3 of BRUKINSA in combination with venetoclax for patients with del(17p) or TP53 mutations were reviewed at ASH
CAMBRIDGE, Mass. & BEIJING–(BUSINESS WIRE)–$BGNE #BGNE–BeiGene (NASDAQ: BGNE; HKEX: 06160), a global, science-driven biotechnology company focused on developing innovative and affordable medicines to improve treatment outcomes and access for patients worldwide, today announced results from a planned interim analysis of the Phase 3 SEQUOIA trial in patients with treatment-naïve (TN) chronic lymphocytic leukemia (CLL), including the randomized Cohort 1 comparing BRUKINSA to bendamustine plus rituximab (B+R) and Cohort 3 (Arm D) of BRUKINSA in combination with venetoclax in patients with deletion of chromosome 17p (del[17p]) and/or pathogenic TP53 variants. These data were reported in two oral presentations at the 63rd American Society for Hematology (ASH) Annual Meeting.
“In the positive SEQUOIA trial, BRUKINSA delivered the therapeutic promise of a selective BTK inhibitor as a frontline treatment for CLL patients, with demonstrated superiority over chemoimmunotherapy. These robust data, along with the results from our previously reported Phase 3 ALPINE trial, strengthen our belief that BRUKINSA could become an important new treatment option for patients with CLL,” remarked Jane Huang, M.D., Chief Medical Officer of Hematology at BeiGene. “In addition, BRUKINSA achieved favorable safety advantages in both trials such as lower rates of atrial fibrillation.”
“Compared to chemoimmunotherapy, BRUKINSA demonstrated superior PFS benefit for CLL patients receiving frontline treatment, including those harboring high-risk characteristics, such as unmutated IGHV status and del(11q),” said Constantine Tam, MBBS, M.D., Peter MacCallum Cancer Center, and a principal investigator of the study. “Safety findings in SEQUOIA were similar to what has been reported in other BRUKINSA clinical trials, with consistently low rates of atrial fibrillation. Based on these results, BRUKINSA, as a highly selective BTK inhibitor, can potentially provide a chemo-free treatment option for CLL patients.”
For more information on BeiGene’s clinical program and company updates, please visit BeiGene’s virtual booth at this year’s ASH Annual Meeting at http://www.beigenevirtualexperience.com.
SEQUOIA Cohort 1: BRUKINSA vs. B+R in TN CLL Patients Without del (17p)
Oral Presentation; Abstract #396; Plain language summary available at https://www.beigene.com/pls/ash2021/sequoia
A total of 479 patients with TN CLL whose tumor did not exhibit del(17p) were enrolled in Cohort 1 of the SEQUOIA trial, with 241 patients randomized to receive BRUKINSA (Arm A) and 238 patients randomized to receive B+R (Arm B). Patient characteristics were balanced between the two arms, with more than 50% with unmutated IGHV gene and 18% with del(11q) in each. Patients with del(17p) typically have poor response to chemoimmunotherapy and were assigned to receive BRUKINSA treatment in Cohort 2. Results from Cohort 2 were previously presented at the 2020 ASH Annual Meeting.
The primary endpoint of the SEQUOIA trial is progression-free survival (PFS) per independent review committee (IRC) assessment in the randomized Cohort 1.
At the interim analysis, with a median follow-up of 26.15 months, BRUKINSA demonstrated superiority in PFS over B+R, as assessed by IRC. Results included:
- The 24-month PFS rate was 85.5% (95% CI: 80.1, 89.6) in Arm A, compared to 69.5% (95% CI: 62.4, 75.5) in Arm B, with a hazard ratio (HR) of 0.42 (95% CI: 0.27, 0.63), p < 0.0001;
- PFS benefit was consistently observed across key patient subgroups, including patients with del(11q), unmutated IGHV status, Binet stage C, and bulky disease; and
- Overall survival (OS) results were early, and at 24 months, OS probability was similar between two arms, with 94.3% (95% CI: 90.4, 96.7) in Arm A and 94.6% (95% CI: 90.6, 96.9) in Arm B.
Safety analysis was based on 240 patients in Arm A and 227 patients in Arm B who received at least one dose of respective treatment. BRUKINSA was generally well tolerated with a safety profile consistent with its broad clinical program, including a low rate of atrial fibrillation. Results included:
- 224 patients (93.3%) in Arm A experienced at least one adverse event (AE) of any grade, with the most common (≥12%) being contusion (19.2%), upper respiratory tract infection (17.1%), neutropenia (15.4%), diarrhea (13.8%), and arthralgia (13.3%);
- In comparison, 218 patients (96.0%) in Arm B experienced at least one AE of any grade, with the most common (≥12%) being neutropenia (56.8%), nausea (32.6%), pyrexia (26.4%), rash (19.4%), anemia (18.9%), constipation (18.9%), infusion-related reaction (18.9%), fatigue (15.9%), vomiting (14.5%), thrombocytopenia (13.7%), and diarrhea (13.2%);
- 126 patients (52.5%) in Arm A experienced at least one Grade ≥3 AE, compared to 181 patients (79.7%) in Arm B, with the most common in both arms being neutropenia (11.3% in Arm A vs. 51.1% in Arm B) and thrombocytopenia (1.7% in Arm A vs. 7.0% in Arm B);
- 88 patients (36.7%) in Arm A experienced at least one serious AE, compared to 113 patients (49.8%) in Arm B;
- AEs leading to dose reduction, interruption or delay, and discontinuation occurred in 18 patients (7.5%), 111 patients (46.3%), and 20 patients (8.3%), respectively, in Arm A, compared to 84 patients (37.4%), 154 patients (67.8%), and 31 patients (13.7%), respectively, in Arm B;
- Fatal AEs were reported in 11 patients (4.6%) in Arm A, compared to 11 patients (4.8%) in Arm B;
- AEs of interest of any grade included anemia (Arm A vs. Arm B: 4.6% vs. 19.4%), arthralgia (13.3% vs. 8.8%), atrial fibrillation (3.3% vs. 2.6%), bleeding (45.0% vs. 11.0%), diarrhea (13.8% vs. 13.7%), hypertension (14.2% vs. 10.6%), infections (62.1% vs. 55.9%), myalgia (3.8% vs. 1.3%), neutropenia (15.8% vs. 56.8%), other cancers (12.9% vs. 8.8%), and thrombocytopenia (4.6% vs. 17.6%).
In addition, efficacy results with an extended follow-up from Cohort 2 (Arm C) of BRUKINSA as a monotherapy in patients with del(17p) were reported at ASH. With a median follow-up of 30.5 months, the 24-month PFS rate was 88.9% (95% CI: 81.3, 93.6).
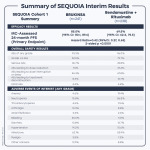
Summary of SEQUOIA Cohort 1 Interim Analysis
| SEQUOIA Cohort 1 Summary | BRUKINSA (n=241) |
| Bendamustine + Rituximab (n=238) | |
| Efficacy Results | ||||
| IRC-Assessed 24-month PFS (Primary Endpoint) | 85.5% (95% CI: 80.1, 89.6) |
| 69.5% (95% CI: 62.4, 75.5) | |
| Hazard Ratio=0.42 (95%CI: 0.27, 0.63) 2-sided p <0.0001 | ||||
| Overall Safety Results | ||||
| AEs of any grade | 93.3% |
| 96.0% | |
| Grade ≥3 AEs | 52.5% |
| 79.7% | |
| Serious AEs | 36.7% |
| 49.8% | |
| AEs leading to dose reduction | 7.5% |
| 37.4% | |
| AEs leading to dose interruption or delay | 46.3% |
| 67.8% | |
| AEs leading to treatment discontinuation | 8.3% |
| 13.7% | |
| Fatal AEs | 4.6% |
| 4.8% | |
| Adverse Events of Interest (Any Grade) | ||||
| Anemia | 4.6% |
| 19.4% | |
| Neutropenia | 15.8% |
| 56.8% | |
| Thrombocytopenia | 4.6% |
| 17.6% | |
| Arthralgia | 13.3% |
| 8.8% | |
| Atrial fibrillation | 3.3% |
| 2.6% | |
| Bleeding | 45.0% |
| 11.0% | |
| Diarrhea | 13.8% |
| 13.7% | |
| Hypertension | 14.2% |
| 10.6% | |
| Infections | 62.1% |
| 55.9% | |
| Myalgia | 3.8% |
| 1.3% | |
| Other cancers | 12.9% |
| 8.8% | |
SEQUOIA Cohort 3 (Arm D): BRUKINSA + Venetoclax in TN CLL Patients with del(17p) and/or TP53 Mutations
Oral Presentation; Abstract #67
Cohort 3 of SEQUOIA was designed to examine the hypothesis that the addition of venetoclax to BRUKINSA can drive tumors into deeper remission. Building on the demonstrated efficacy and safety of BRUKINSA in Cohort 2, Cohort 3 is planned to enroll approximately 80 patients with TN CLL whose tumor exhibits del(17p) or TP53 mutations, with key endpoints being safety, overall response rate (ORR), PFS, and duration of response (DoR). These patients will receive BRUKINSA treatment at 160 mg twice daily for three months, followed by combination treatment of BRUKINSA at the same dosing and venetoclax with a ramp-up dosing to 400 mg once daily for 12 to 24 cycles until progressive disease, unacceptable toxicity, or confirmed undetectable measurable residual disease (uMRD).
“Unfavorable prognosis is often seen in CLL patients with del(17p) or pathogenic TP53 variants, even in the front-line setting. While the follow-up in Cohort 3 was relatively short, the high response rate and the deepened responses observed among those treated for longer periods suggested the potential of BRUKINSA in combination with venetoclax in these high-risk CLL patients. The combination treatment also appeared generally well tolerated,” commented Alessandra Tedeschi, M.D., Grande Ospedale Metropolitano Niguarda in Italy, a principal investigator on the study. “We look forward to the continued evaluation of BRUKINSA in combination with venetoclax in untreated CLL patients with del(17p) or TP53 mutations.”
At the data cutoff on September 7, 2021, 49 patients were enrolled in Cohort 3, including 46 patients (93.9%) with centrally confirmed positive del(17p) status and three patients (6.1%) with a pathogenic TP53 variant alone. Patients enrolled in Cohort 3 also exhibited other markers of high risk, including 87.8% with unmutated IGHV, 91.9% with concurrent TP53 mutation, and 83.3% with complex karyotype (at least three abnormalities).
With a short median follow-up of 12.0 months, a high ORR was observed in the 36 patients who had at least one post-baseline response evaluation by the data cutoff date. Preliminary efficacy results per investigator assessment included:
- Of the 14 patients who received combination treatment for more than 12 months, five patients (36%) achieved a confirmed complete response (CR) or CR with incomplete bone marrow recovery (CRi) in a bone marrow assessment and four additional patients met the criteria for CR or CRi but not confirmed in bone marrow assessment due to COVID-19 restrictions; and
- In all 36 patients evaluable for efficacy, the ORR was 97.2% (95% CI: 85.5, 99.9) and the CR/CRi rate was 13.9% (all CRs or CRis were in patients who received combination treatment for more than 12 months).
With a median follow-up of 7.9 months, safety results in all 49 enrolled patients included:
- 40 patients (81.6%) experienced at least one AE of any grade, with the most common (≥12%) being infections (16.3%), neutropenia (14.3%), bruising (12.2%), diarrhea (12.2%), minor bleeding (12.2%), and nausea (12.2%);
- 16 patients (32.7%) experienced at least one Grade ≥3 AE and four patients (8.2%) experienced at least one serious AE;
- AEs leading to dose interruption, dose reduction, and treatment discontinuation occurred in 10 patients (20.4%), no patients (0.0%), and one patient (2.0%), respectively; and
- One patient (2.0%) experienced a fatal AE.
With a median follow-up of 13.5 months, safety results in the 34 patients who received combination treatment included:
- 29 patients (85.3%) experienced at least one AE of any grade, with the most common (≥12%) being infections (23.5%), neutropenia (20.6%), diarrhea (14.7%), fatigue (14.7%), nausea (14.7%), and bruising (11.8%);
- 13 patients (38.2%) experienced at least one Grade ≥3 AE and three patients (8.8%) experienced at least one serious AE; and
- AEs leading to dose interruption occurred in 10 patients (29.4%), with no AEs leading to dose reduction or treatment discontinuation.
About SEQUOIA
SEQUOIA is a randomized, multicenter, global Phase 3 trial (NCT03336333) designed to evaluate the efficacy and safety of BRUKINSA compared to B+R in patients with TN CLL or SLL. The trial consists of three cohorts:
- Cohort 1 (n=479): randomized 1:1 to receive BRUKINSA (n=241) or B+R (n=238) until disease progression or unacceptable toxicity, in patients not harboring del(17p); data from this group comprise the primary endpoint;
- Cohort 2 (n=110): patients with del(17p) receiving BRUKINSA as a monotherapy; and
- Cohort 3 (enrollment ongoing): patients with del(17p) or pathogenic TP53 variant receiving BRUKINSA in combination with venetoclax.
Patients with del(17p) were not randomized to B+R, as they experience poor clinical outcomes and poor response to chemoimmunotherapy. The primary endpoint of the trial is IRC-assessed PFS. Secondary endpoints include investigator-assessed PFS, IRC- and investigator-assessed overall response rate (ORR), overall survival (OS), PFS and ORR in patients with del(17p), and safety.
Cohort 2 (Arm C), representing high-risk patients treated with BRUKINSA monotherapy, was previously presented at the American Society for Hematology (ASH) Annual Meeting in December 2020. This cohort of patients with del(17p) achieved significant efficacy with an 18-month PFS of 90.6%, as assessed by investigator.
About Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma
Chronic lymphocytic leukemia (CLL) is the most common form of leukemia in adults, with a global incidence of approximately 114,000 new cases in 2017.1,2 CLL affects white blood cells or lymphocytes in the bone marrow.1 Proliferation of cancer cells (leukemia) in the marrow result in reduced ability to fight infection and spread into the blood, which affects other parts of the body including the lymph nodes, liver and spleen.1,3 The BTK pathway is a known route that signals malignant B cells and contributes to the onset of CLL.4 Small lymphocytic lymphoma (SLL) is a non-Hodgkin’s lymphoma affecting the B-lymphocytes of the immune system, which shares many similarities to CLL but with cancer cells found mostly in lymph nodes.5
About BRUKINSA
BRUKINSA is a small molecule inhibitor of Bruton’s tyrosine kinase (BTK) discovered by BeiGene scientists that is currently being evaluated globally in a broad clinical program as a monotherapy and in combination with other therapies to treat various B-cell malignancies. Because new BTK is continuously synthesized, BRUKINSA was specifically designed to deliver complete and sustained inhibition of the BTK protein by optimizing bioavailability, half-life, and selectivity. With differentiated pharmacokinetics compared to other approved BTK inhibitors, BRUKINSA has been demonstrated to inhibit the proliferation of malignant B cells within a number of disease relevant tissues.
BRUKINSA has received 12 approvals covering 40 countries and regions:
- For the treatment of mantle cell lymphoma (MCL) in adult patients who have received at least one prior therapy (United States, November 2019)*;
- For the treatment of MCL in adult patients who have received at least one prior therapy (China, June 2020)**;
- For the treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in adult patients who have received at least one prior therapy (China, June 2020)**;
- For the treatment of relapsed or refractory MCL (United Arab Emirates, February 2021);
- For the treatment of Waldenström’s macroglobulinemia (WM) in adult patients (Canada, March 2021);
- For the treatment of adult patients with WM who have received at least one prior therapy (China, June 2021)**;
- For the treatment of MCL in adult patients who have received at least one prior therapy (Canada, July 2021);
- For the treatment of MCL in adult patients who have received at least one prior therapy (Chile, July 2021);
- For the treatment of adult patients with MCL who have received at least one previous therapy (Brazil, August 2021);
- For the treatment of adult patients with WM (United States, August 2021);
- For the treatment of adult patients with marginal zone lymphoma (MZL) who have received at least one anti-CD20-based regimen (United States, September 2021)*;
- For the treatment of adult patients with MCL who have received at least one previous therapy (Singapore, October 2021);
- For the treatment of MCL in patients who have received at least one prior therapy (Israel, October 2021);
- For the treatment of adult patients with WM who have received at least one prior therapy, or in first line treatment for patients unsuitable for chemo-immunotherapy (Australia, October 2021);
- For the treatment of adult patients with MCL who have received at least one prior therapy (Australia, October 2021);
- For the treatment of adult patients with MCL who have received at least one previous therapy (Russia, October 2021);
- For the treatment of adult patients with MCL who have received at least one previous therapy (Saudi Arabia, November 2021); and
- For the treatment of adult patients with WM who have received at least one prior therapy or first-line treatment of patients unsuitable for chemo-immunotherapy (European Union plus Iceland and Norway, November 2021).
To date, more than 20 marketing authorization applications have been submitted for BRUKINSA for various indications.
* This indication was approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
** This indication was approved under conditional approval. Complete approval for this indication may be contingent upon results from ongoing randomized, controlled confirmatory clinical trials.
IMPORTANT U.S. SAFETY INFORMATION FOR BRUKINSA (ZANUBRUTINIB)
Warnings and Precautions
Hemorrhage
Fatal and serious hemorrhagic events have occurred in patients with hematological malignancies treated with BRUKINSA monotherapy. Grade 3 or higher hemorrhage including intracranial and gastrointestinal hemorrhage, hematuria and hemothorax have been reported in 3.4% of patients treated with BRUKINSA monotherapy. Hemorrhage events of any grade occurred in 35% of patients treated with BRUKINSA monotherapy.
Bleeding events have occurred in patients with and without concomitant antiplatelet or anticoagulation therapy. Co-administration of BRUKINSA with antiplatelet or anticoagulant medications may further increase the risk of hemorrhage.
Monitor for signs and symptoms of bleeding. Discontinue BRUKINSA if intracranial hemorrhage of any grade occurs. Consider the benefit-risk of withholding BRUKINSA for 3-7 days pre- and post-surgery depending upon the type of surgery and the risk of bleeding.
Infections
Fatal and serious infections (including bacterial, viral, or fungal) and opportunistic infections have occurred in patients with hematological malignancies treated with BRUKINSA monotherapy. Grade 3 or higher infections occurred in 27% of patients, most commonly pneumonia. Infections due to hepatitis B virus (HBV) reactivation have occurred.
Consider prophylaxis for herpes simplex virus, pneumocystis jiroveci pneumonia and other infections according to standard of care in patients who are at increased risk for infections. Monitor and evaluate patients for fever or other signs and symptoms of infection and treat appropriately.
Cytopenias
Grade 3 or 4 cytopenias, including neutropenia (26%), thrombocytopenia (11%) and anemia (8%) based on laboratory measurements, developed in patients treated with BRUKINSA monotherapy. Grade 4 neutropenia occurred in 13% of patients, and Grade 4 thrombocytopenia occurred in 3.6% of patients.
Monitor complete blood counts regularly during treatment and interrupt treatment, reduce the dose, or discontinue treatment as warranted. Treat using growth factor or transfusions, as needed.
Second Primary Malignancies
Second primary malignancies, including non-skin carcinoma, have occurred in 14% of patients treated with BRUKINSA monotherapy. The most frequent second primary malignancy was non-melanoma skin cancer, reported in 8% of patients. Other second primary malignancies included malignant solid tumors (4.0%), melanoma (1.7%) and hematologic malignancies (1.2%). Advise patients to use sun protection and monitor patients for the development of second primary malignancies.
Cardiac Arrhythmias
Atrial fibrillation and atrial flutter were reported in 3.2% of patients treated with BRUKINSA monotherapy. Patients with cardiac risk factors, hypertension, and acute infections may be at increased risk. Grade 3 or higher events were reported in 1.1% of patients treated with BRUKINSA monotherapy. Monitor signs and symptoms for atrial fibrillation and atrial flutter and manage as appropriate.
Embryo-Fetal Toxicity
Based on findings in animals, BRUKINSA can cause fetal harm when administered to a pregnant woman. Administration of zanubrutinib to pregnant rats during the period of organogenesis caused embryo-fetal toxicity including malformations at exposures that were 5 times higher than those reported in patients at the recommended dose of 160 mg twice daily. Advise women to avoid becoming pregnant while taking BRUKINSA and for 1 week after the last dose. Advise men to avoid fathering a child during treatment and for 1 week after the last dose.
If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
Adverse reactions
The most common adverse reactions, including laboratory abnormalities, in ≥ 30% of patients who received BRUKINSA (N = 847) included decreased neutrophil count (54%), upper respiratory tract infection (47%), decreased platelet count (41%), hemorrhage (35%), decreased lymphocyte count (31%), rash (31%) and musculoskeletal pain (30%).
Drug Interactions
CYP3A Inhibitors: When BRUKINSA is co-administered with a strong CYP3A inhibitor, reduce BRUKINSA dose to 80 mg once daily. For coadministration with a moderate CYP3A inhibitor, reduce BRUKINSA dose to 80 mg twice daily.
CYP3A Inducers: Avoid coadministration with moderate or strong CYP3A inducers.
Specific Populations
Hepatic Impairment: The recommended dose of BRUKINSA for patients with severe hepatic impairment is 80 mg orally twice daily.
Please see full U.S. Prescribing Information at www.beigene.com/PDF/BRUKINSAUSPI.pdf and Patient Information at www.beigene.com/PDF/BRUKINSAUSPPI.pdf.
BeiGene Oncology
BeiGene is committed to advancing best and first-in-class clinical candidates internally or with like-minded partners to develop impactful and affordable medicines for patients across the globe. We have a growing R&D team of approximately 2,750 colleagues dedicated to advancing more than 90 ongoing or planned clinical trials that have involved more than 14,000 patients and healthy volunteers. Our expansive portfolio is directed predominantly by our internal colleagues supporting clinical trials in more than 45 countries and regions. Hematology-oncology and solid tumor targeted therapies and immuno-oncology are key focus areas for the Company, with both mono- and combination therapies prioritized in our research and development. BeiGene currently has three approved medicines discovered and developed in our own labs: BTK inhibitor BRUKINSA in the United States, China, the EU, Canada, Australia and additional international markets; and the non-FC-gamma receptor binding anti-PD-1 antibody tislelizumab as well as the PARP inhibitor pamiparib in China.
BeiGene also partners with innovative companies who share our goal of developing therapies to address global health needs. We commercialize a range of oncology medicines in China licensed from Amgen and Bristol Myers Squibb. We also plan to address greater areas of unmet need globally through our collaborations including with Amgen, Bio-Thera, EUSA Pharma, Mirati Therapeutics, Seagen, and Zymeworks.
Contacts
BeiGene
Investor Contact
Gabrielle Zhou
+86 10-5895-8058
[email protected]
Media Contact
Vivian Ni
+1 857-302-7596
[email protected]




